Introduction
Myelodysplastic syndromes (MDS) affect mainly older individuals, with a median age > 70 years. Reduced intensity conditioning (RIC) is usually used for MDS patients undergoing allo-HCT to balance the toxicity of myeloablative conditioning (MAC), particularly in the more elderly or those with multiple comorbidities. In addition, the clinicians subjective opinion/ experience remains a major guide in choosing the intensity of conditioning. The majority of studies comparing RIC and MAC reported a higher cumulative incidence (CI) of relapse (RP) and lower non-relapse mortality (NRM) in the RIC groups. However, the impact of conditioning intensity on outcome after allo-HCT remains controversial. To gain more insight into the impact of conditioning on outcomes following allo-HCT for MDS, we evaluated RIC versus MAC in MDS patients aged > 50 years in a large EBMT cohort.
Patients and methods
This was a registry-based retrospective multicenter study that included MDS patients above 50 years, who received a first allo-HCT following RIC or MAC conditioning between 2014 - 2018 and had data on IPSS-R at allo-HCT and RIC/MAC conditioning available. Data collected included disease features, patient, donor and transplantation characteristics. Patients with ex vivo T-cell depletion were excluded. Variables with missing values ≤35% were handled with multiple imputation. OS, DFS, RP and NRM were compared using the Log-rank and Gray's test for CI, and (cause-specific) Cox proportional hazard models for multivariable analyses (MVA). A conditional logistic regression after propensity score matching (PSM) was also performed for potential risk confounders.
Results
Among the 1393 included patients (from 121 centres), 922 (66%) were men and the median age at allo-HCT was 62.8 (IQR:58.2-66.9) years. The majority of patients (n=884; 64.3%) had RAEB. The IPSS-R was recorded as very low/low (n=598, 43%), intermediate (n=352, 25%) and high/very high (n=443, 32%). Cytogenetic risk score was very good/good (n=932, 66.9%), intermediate (n=250, 17.9%) and poor/very poor (n=211, 15.1%). Karnofsky index was ≥ 90 in 916 pts (69.3%) and HCT-CI ≥ 3 in 292 pts (27.3%). Disease status at transplant was recorded as complete remission (n=486, 34.9%), untreated/stable disease (n=544, 39.0%), and progressive disease (n=310, 22.3%). Donor was HLA-matched (related/unrelated) (n=989, 71.1%), unrelated HLA-mismatched (n=286, 20.5%) or familial HLA haplo-identical donor (n=153, 10.9%). Source of SC was BM (n=112, 8.0%) and PB (n=1255, 90.0%). A RIC regimen was used in 1053 (75.5%) patients. In vivo T-cell depletion with anti-thymocyte globulin (ATG) was used in 941 pts (67.5%).
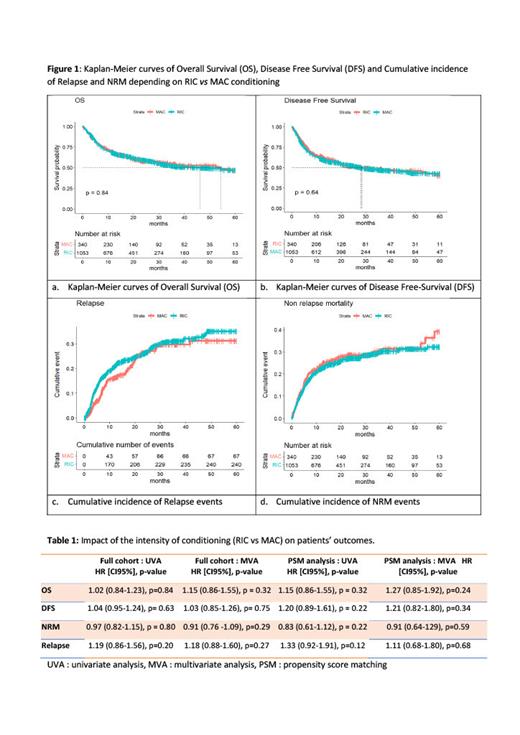
Median time of follow up was 27.9 months (IQR: 26.4-30.6). Median rate of OS in RIC vs MAC group was 54.2(95% CI: 33.5-NA) vs 46.2(95% CI: 32.6-69.4), p=0.84; median rate of DFS was 28.4(95% CI: 19.4-58) vs 28.0 (95% CI: 22.5-39.1), respectively, p=0.64. Cumulative incidence rate of RP and NRM for RIC vs MAC regimen at 36 months were 31.2% vs 29.7% and 29.9% vs 30.4%, respectively (Figure 1). Both in univariable and MVA we did not observe a significant (> 0.05) association between the conditioning regimen on the outcomes. Similar results were obtained using PSM to control potential confounders (such as age, source of SC, stage of disease, HCT-CI, cytogentic risk score, neutrophile and platelet counts and sAML) and conditional logistic regression analysis (Table1).
Conclusion
To our knowledge, this is the largest retrospective cohort study that highlights a lack of association between RIC/MAC regimen and outcomes in MDS patients undergoing allo-HCT. Our results are in line with the recent published systematic review and metanalysis where evidence for using a one conditioning regimen over another remains weak [1,2].
1) Rashidi et al. Biology of Blood and Marrow Transplantation. 2020; 26:138-141.
2) Akbar et al. Blood.2020; 136:40-41.
Disclosures
Peffault De Latour:Jazz Pharmaceuticals: Honoraria. Chevallier:Immedica Pharma: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; Mallinckrodt Pharmaceuticals: Honoraria; Incyte: Honoraria, Research Funding; Servier: Honoraria. McLornan:UK ALL RIC TRIAL - DSM board: Other: participation on a data safety monitoring board or advisory board; Novartis: Honoraria; Abbvie: Honoraria; Jazz Pharma: Honoraria; EBMT Scientific Council Member: Other: Chair of EBMT CMWP; Imago Biosciences: Research Funding. Yakoub-Agha:Kite, a Gilead Company: Honoraria, Other: travel support; Janssen: Honoraria; Novartis: Honoraria; Bristol Myers Squibb: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal